
Inhibition of Fe2+- and Fe3+- induced hydroxyl radical production by the iron-chelating drug deferiprone - ScienceDirect

Fe2+/Fe3+ Cycling for Coupling Self‐Powered Hydrogen Evolution and Preparation of Electrode Catalysts - Chen - 2022 - Angewandte Chemie International Edition - Wiley Online Library

Fe2+/Fe3+ Ions Chelated with Ultrasmall Polydopamine Nanoparticles Induce Ferroptosis for Cancer Therapy | ACS Biomaterials Science & Engineering

Schematic of intestinal iron uptake. Fe3+ in the intestinal lumen is... | Download Scientific Diagram

Enhanced electro-reduction of Fe3+ to Fe2+ by acidified carbon nanotube-modified graphite cathode and its application in a novel Fenton process for p-nitrophenol degradation - ScienceDirect

SOLVED: QUESTion points Save Anawor If a Galvanic cell is created using the Ag+/Ag and Fe2+/Fe3t half reactions, what is the net reaction for this electrochemical cell? Fe3-(aq) Ag"(aq) Fe?+(aq) Agls) Fe?-(aq)

Sketch the voltaic cell containing Zn|Zn2+ and Fe2+|Fe3+ half-cells. Calculate the Ecell. Be sure to label everything. Hint: There has to be a solid state support. | Homework.Study.com

SOLVED: Consider the following voltaic cell: Pt(s)IFe2+ (aq) , Fe3+(aq) Ag" (aq)Ag(s) Identify the half-reaction that occurs at the anode Select one: a. Fe3- (aq) + e Fe2- (aq) b. Pt(s) +

Given standard electrode potentials, Fe^2 + 2e^-→ Fe, E^∘ = - 0.440 V Fe^3 + + 3e^-→ Fe, E^∘ = - 0.036 V The standard electrode potential (E^∘) for Fe^3 + + e^-→ Fe^2 + is:
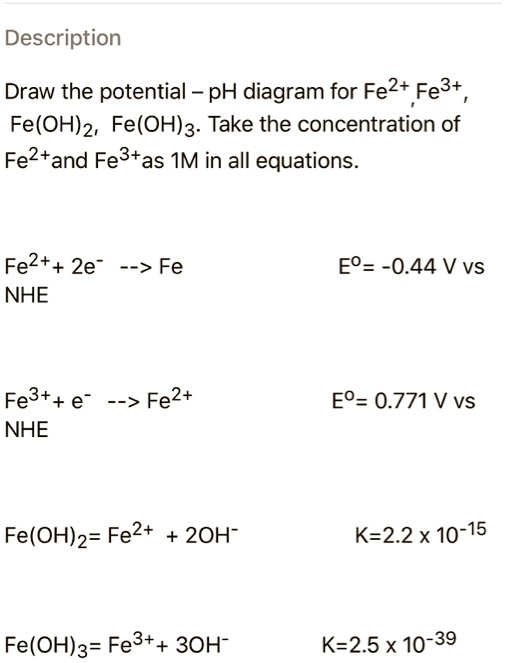





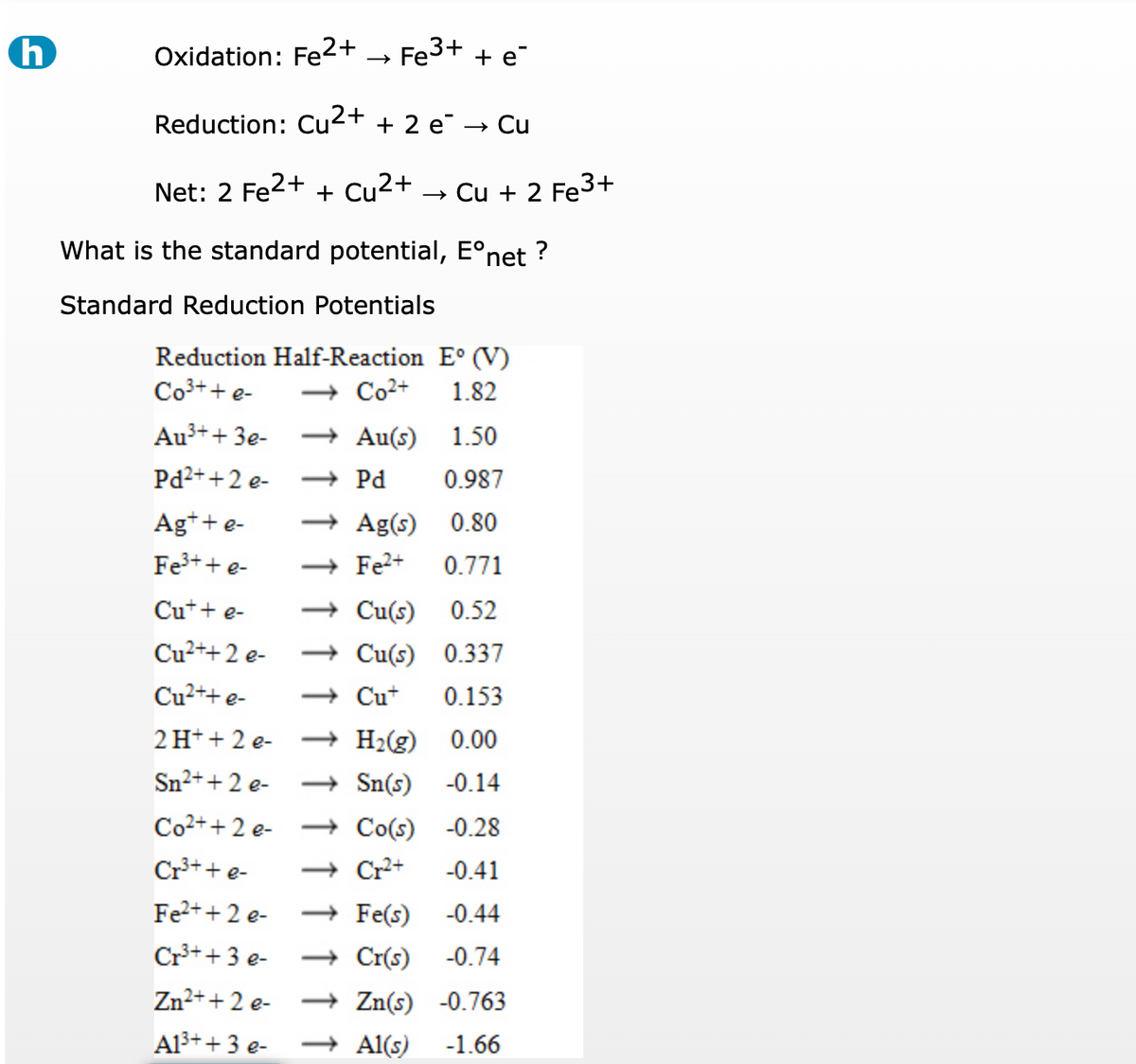
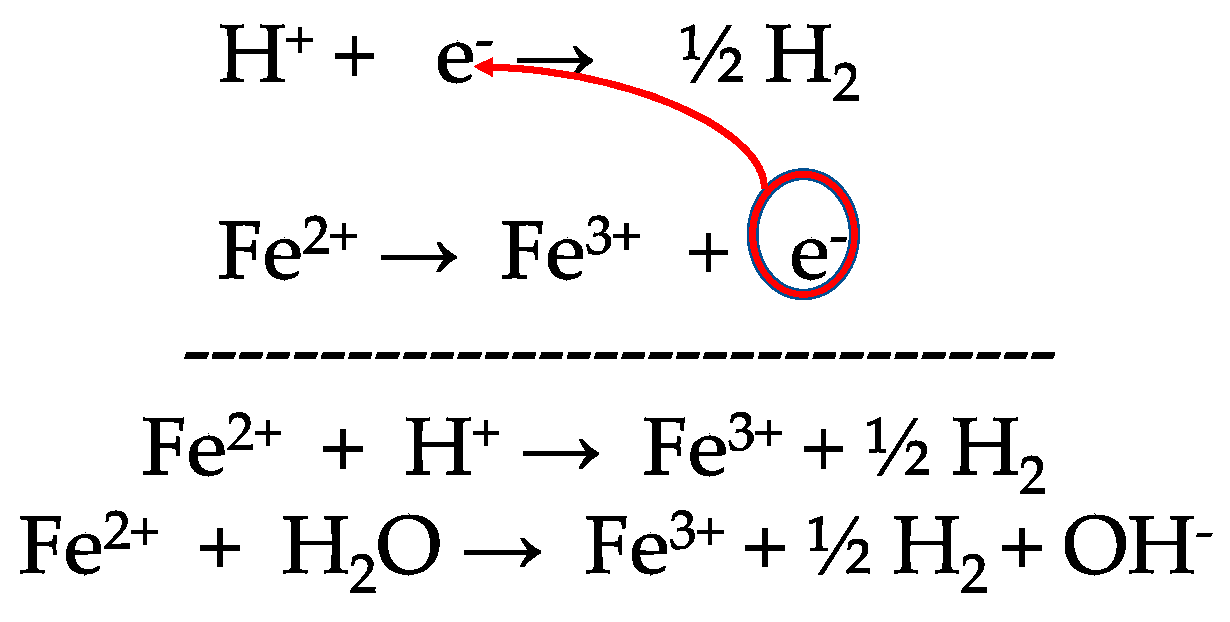

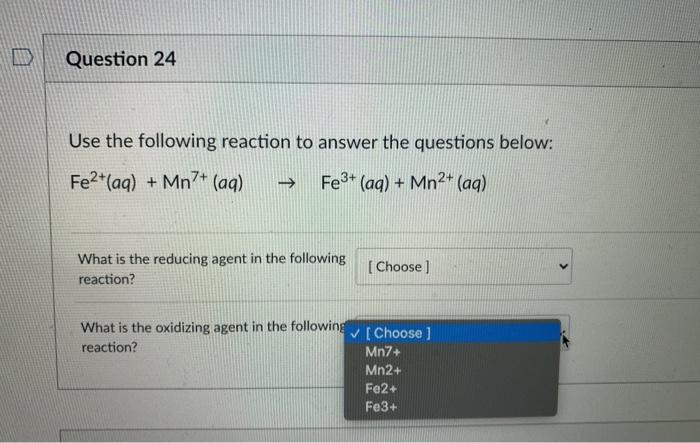
.PNG)