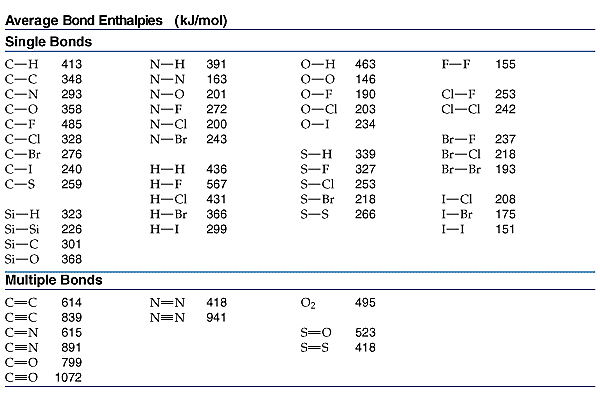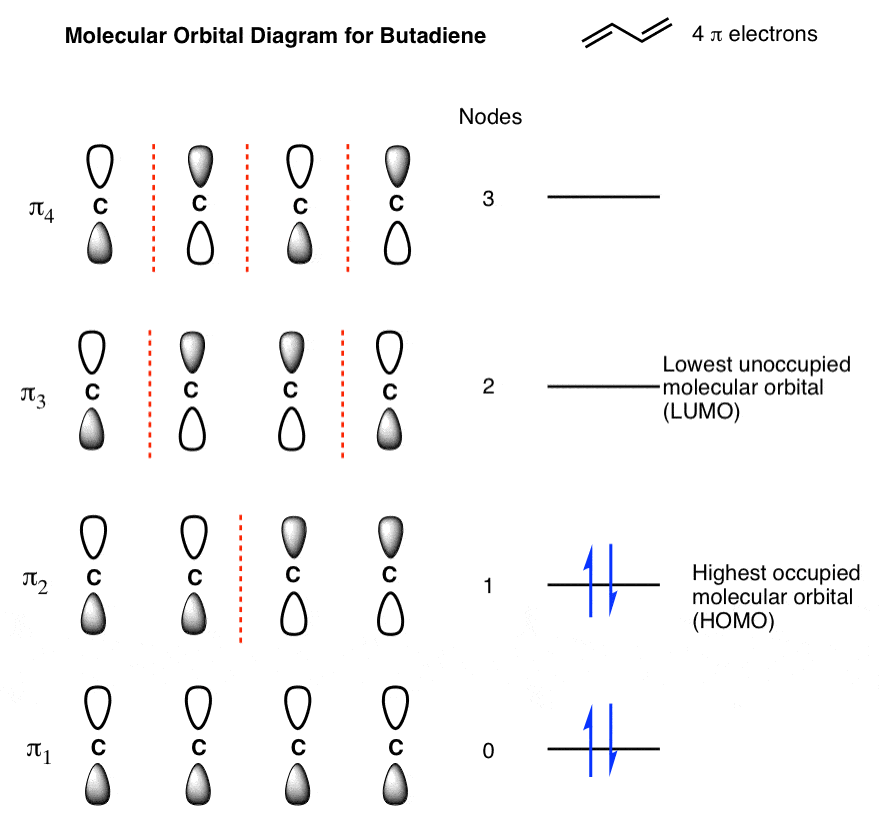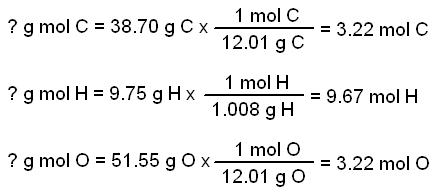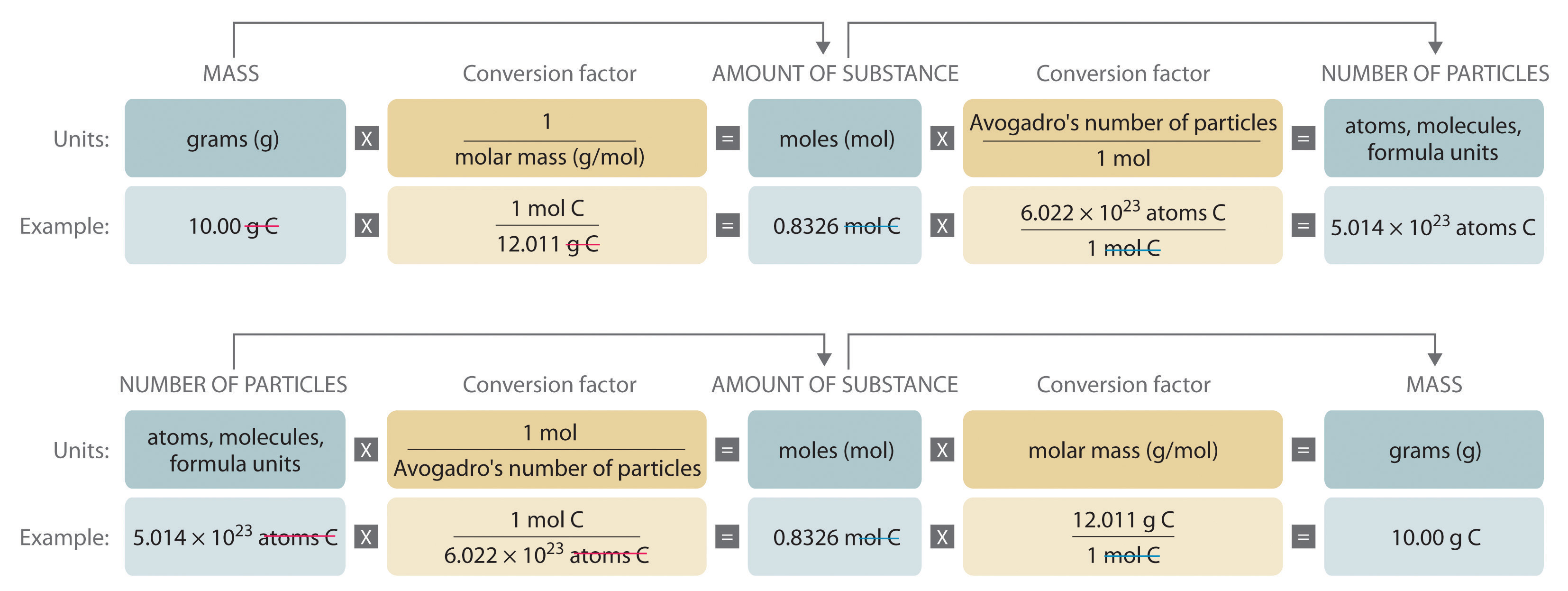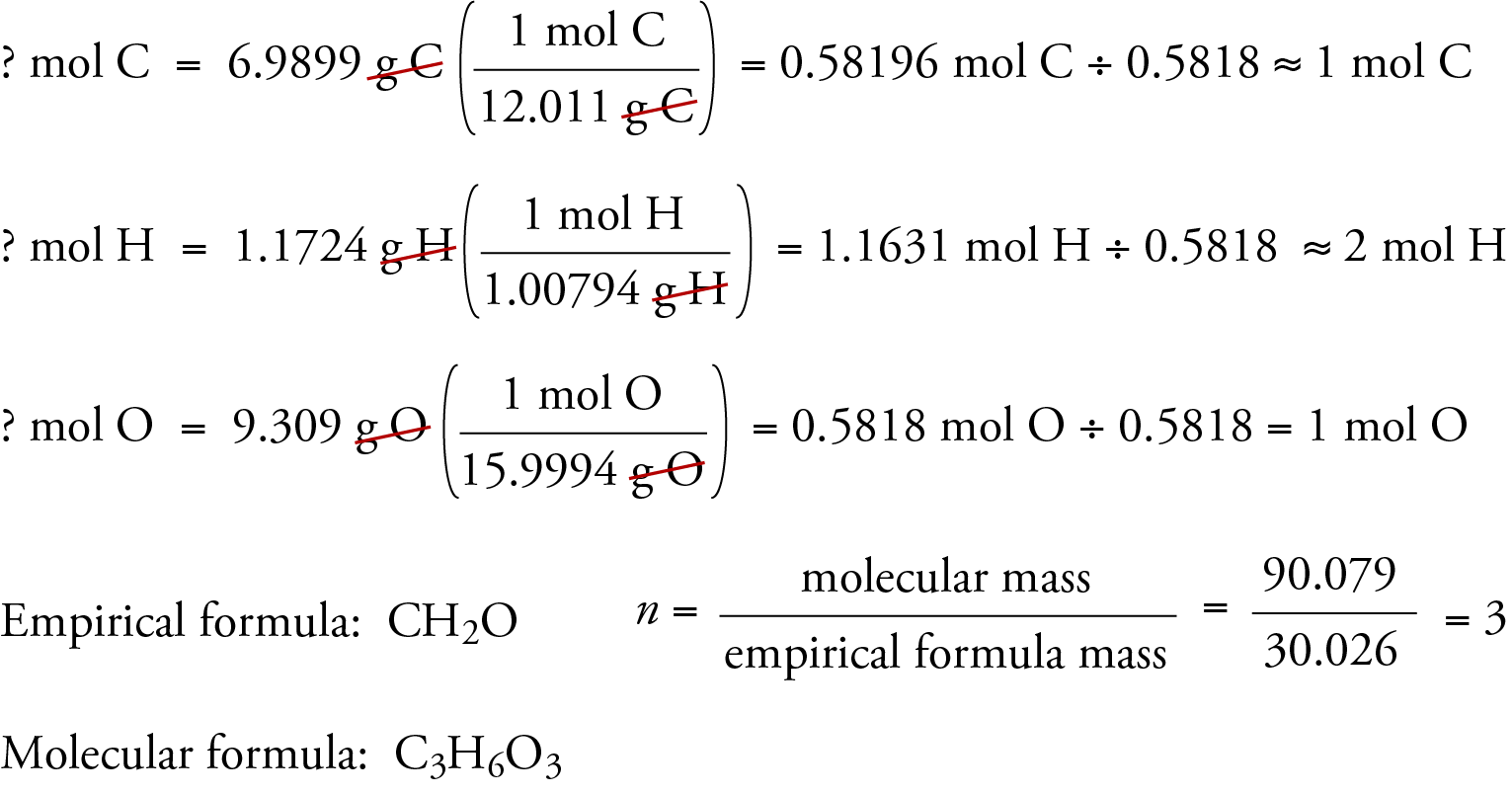
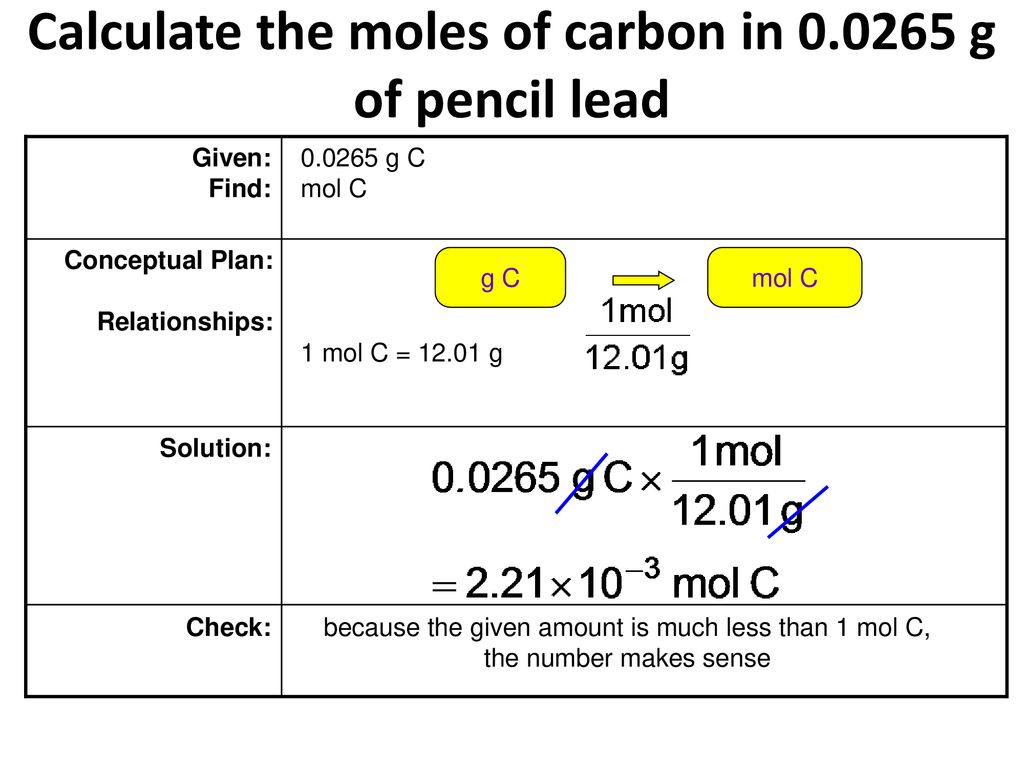
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are
![SOLVED: Conversion Factors 1 gal = 231 in' (exact) 1 atm 760 torr (exact) cm = ] mL (exact) 1 b = 453.59237 g (exact) 2.54 cm = L in (exact) 1 SOLVED: Conversion Factors 1 gal = 231 in' (exact) 1 atm 760 torr (exact) cm = ] mL (exact) 1 b = 453.59237 g (exact) 2.54 cm = L in (exact) 1](https://cdn.numerade.com/ask_images/98c41ed6b3e54a558ac16356f85004bd.jpg)
SOLVED: Conversion Factors 1 gal = 231 in' (exact) 1 atm 760 torr (exact) cm = ] mL (exact) 1 b = 453.59237 g (exact) 2.54 cm = L in (exact) 1
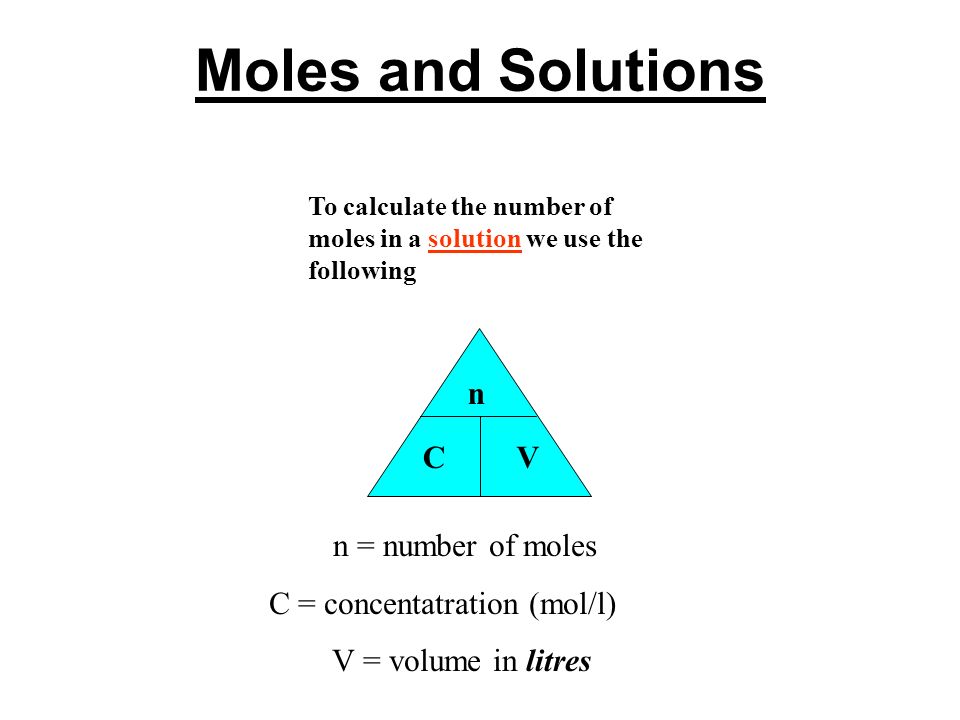
Moles and Solutions g n gfm To calculate the number of moles in a solution we use the following n CV n = number of moles C = concentatration (mol/l) V. - ppt download
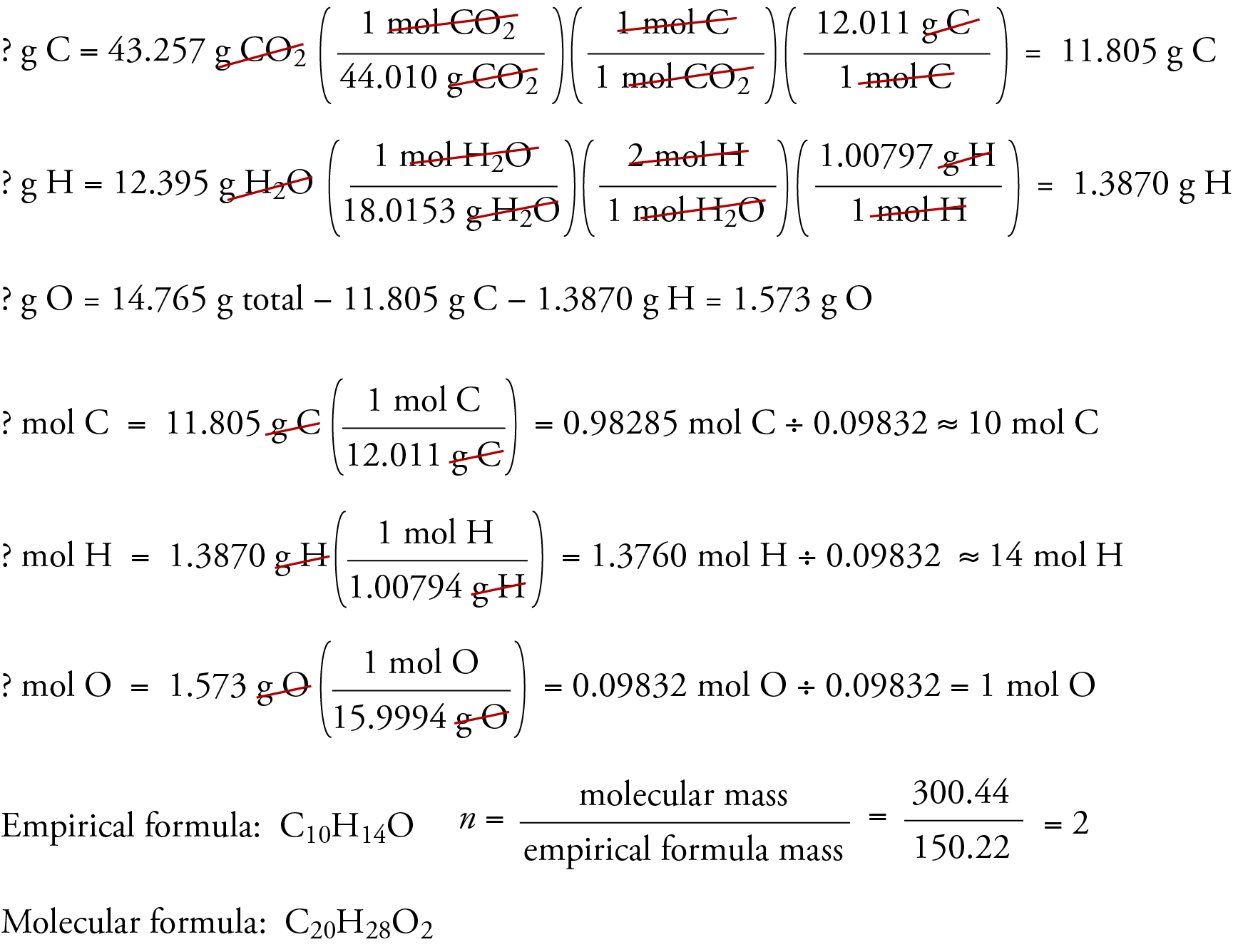
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are
1) At 2257 K and 1.00 atm total pressure, water is 1.77 per cent dissociated at equilibrium by way of the reaction 2 H2O(g) <

Temperature effect (10-55 • C) on the phenolphthalein (PHP);-CD-PHP... | Download Scientific Diagram


:max_bytes(150000):strip_icc()/vitamin-c-molecular-model-483948223-582c8a523df78c6f6a473f1c.jpg)